We are Ethan Chew and Sean Tang from Group E, and we are doing an investigation on the Effect of Reactivity of metals and how it affects the Redox Reaction of a Voltaic Cell.
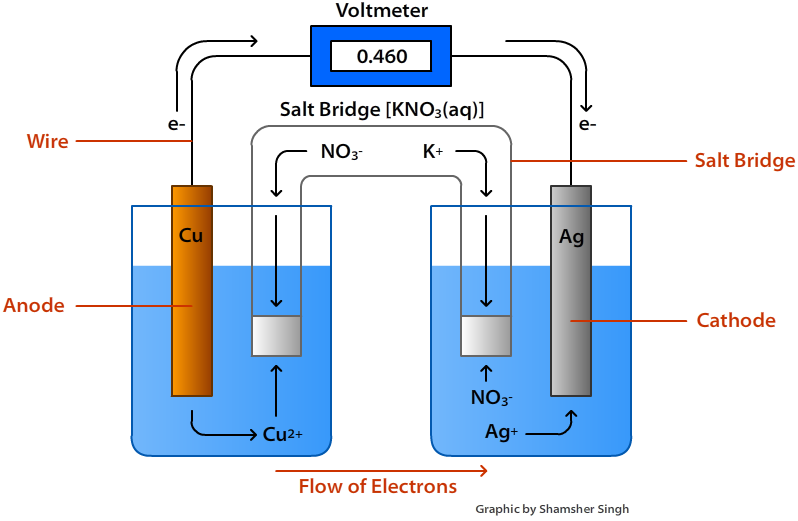
A Voltaic Cell, also known as a Galvanic Cell, is an electrochemical cell that derives electrical energy from Spontaneous Redox Reactions taking place within the cell. Generally, it either consists of two different metals connected by a Salt Bridge. (Wikipedia, n.d)
Fig.1 Photovoltaic Cell
An Oxidation-Reduction (Redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An Oxidation-Reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. (LibreTexts, 2017)
Reduction Potential is a measure of the tendency of a chemical species to acquire or lose electrons and thereby be reduced. Reduction potential is measured in volts (V), or millivolts (mV).
(Wikipedia, n.d)
By conducting this experiment, we will be able to find which of the metals that we have chosen have the highest reduction potential. thus we can deduce which pair of metals is the most suitable for making batteries.